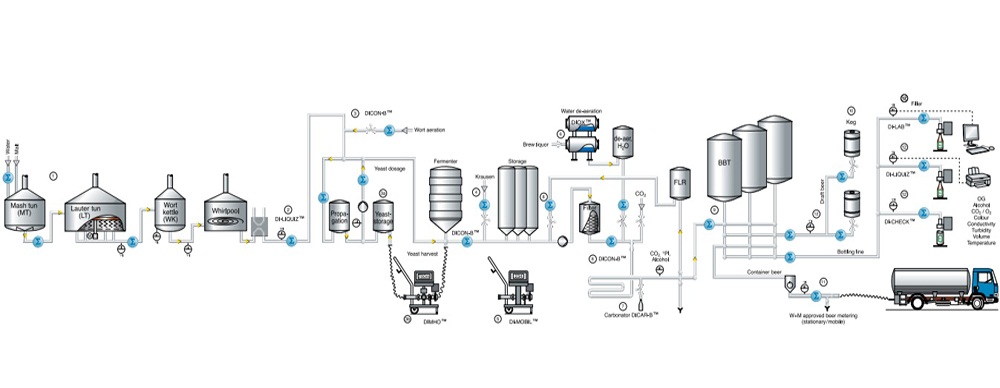
FAT/SAT Support
Our GMP offers a compliant validation package that our customers find a very efficient and time saving resource. This package can include but is not limited to the following:
- GMP Risk Analysis (including workshop with our customer)
- Functional Specifications
- Hardware Specifications
- Software Specifications
- Sensor/Actuator Plans
- Factory Acceptance Test (FAT) Protocols (consisting of critical IQ and OQ elements)
- Site Acceptance Test (SAT) Protocols (consisting of critical IQ and OQ elements)
- Installation Qualification and Operational Qualification Protocols
- Traceability Matrix
The complete validation package consists of documentation only and is supplied in Microsoft Word and Adobe (pdf) files so that customers can expand and re-use these documents for future activities on the projects they are purchased for. Of course custom or special customer requirements for FAT, SAT and Qualification documents can be developed.
BioFluid will gladly support its customers with the performance of validation activities not only at BioFluid but at the customer’s site as well.
- FAT and SAT support/execution
- IQ and OQ support/execution